The ozone/oxygen treatment system is more effective than traditional methods.
09/29/2012
Editor’s Note: This is the last of a four-part series, which provides a comprehensive evaluation of controlling collection system odor and corrosion using onsite oxygen and ozone generation. This series covers the biochemistry of odor generation in the collection system. It also provides background on oxygen and ozone chemistry, which is important to understanding how these gases work. This part of the series discusses the results of the testing that was conducted.
Post-Treatment Analysis
As discussed in Parts 2 and 3, hydrogen sulfide (H2S) levels were elevated. The average H2S indicated was 1,070 parts per million (ppm). Even if the extreme spike above 3,000 ppm were ignored, the average concentration was still about 700 ppm. After ozone/oxygen treatment, concentrations as low as 1 ppm were achieved. However, for calculation purposes, a more realistic average was 7 ppm of H2S, based on OdaLog values following the steep drop, which was considered a treatment induced value. This equates to a 99 percent reduction in H2S levels in a matter of days, with real results starting in about a day (see Figure 1). In Figure 1, the startup of the system is indicated as well as the calculated detention time range, approximately 25 hours. Notice how the H2S concentration began a rapid descent almost exactly at the 25-hour mark (14a). Additionally, the system shutoff was included, and about 25 hours later, the H2S levels began to peak again (14b).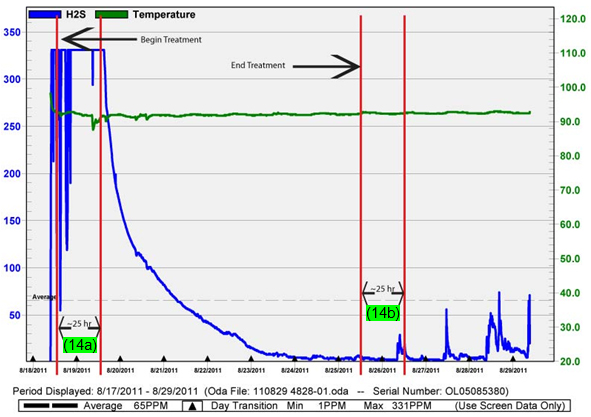 Figure 1. Third-part OdaLog H2S concentrations and response times
Figure 1. Third-part OdaLog H2S concentrations and response times.jpg) Table 1. Third-party grab sample results at MH1
Table 1. Third-party grab sample results at MH1Financial Benefits
Traditional odor control methods employed by utilities and municipalities can create technical and financial compromises, which tend to produce undesirable side effects, including infrastructure corrosion, clogging and the facilitation of the sulfide producing anaerobic slime layer. The use of chemicals also requires ongoing and escalating year-over-year costs. Often, these technical and financial challenges impact performance and compromise results. Using the ozone/oxygen system can quickly and completely control H2S production and increase DO levels. The operational theory is supported by pilot tests. This treatment causes minimal to zero negative side effects. Consider a municipality that uses, on average, 210 gallons of iron salts per day in a force main. For nitrates, the approximate stoichiometric equivalent is about 33 percent less usage than iron, or about 139 gallons per day (gpd). The costs of these methods can be compared to that of an appropriately sized ozone/oxygen system, which would be expected to provide a higher level of overall performance so that an accurate comparison can be made between them, as shown in Figure 2.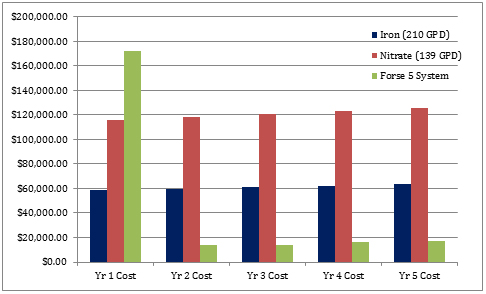 Figure 2. 3- to 5-year annual costs for iron, nitrate and ozone/oxygen treatment; all costs are assumed to increase at a rate of 2 percent annually, which is considered conservative.
Figure 2. 3- to 5-year annual costs for iron, nitrate and ozone/oxygen treatment; all costs are assumed to increase at a rate of 2 percent annually, which is considered conservative.- Iron—210 gpd average usage at $0.75 per gallon
- Nitrate—139 gpd average usage at $2.25 per gallon
- Ozone/oxygen treatment—Capital equipment cost and operating costs of 16 kW at $0.08 per kilowatt hour, 24 hours, 365 days and miscellaneous consumables
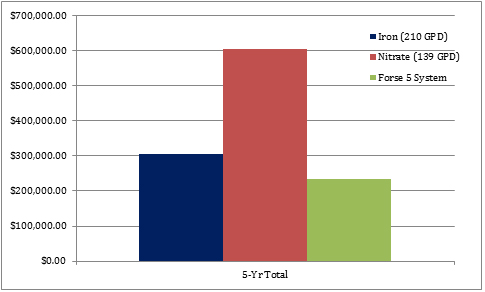 Figure 3. Total 5-year costs for iron, nitrate and ozone/oxygen treatment
Figure 3. Total 5-year costs for iron, nitrate and ozone/oxygen treatmentConclusion
The application of ozone and oxygen into the wastewater environment brings with it a much more technologically and cost-effective way of treatment. It is also more effective from a sustainability standpoint.Series References:
- American Society of Civil Engineers, “ASCE’s Infrastructure Report Card,” ASCE, 2009, http://www.infrastructurereportcard.org/.
- A. Matthews et al, “Control of Hydrogen Sulfide Buildup in Forcemains using Ozone and Oxygen,” Proceedings of the Water Environment Federatin, WEFTEC 2010: Session 101 through Session 112, pp. 7591-7611(21), 2010.
- U.S. Environmental Protection Agency, “Odor and Corrosion Control in Sanitary Sewerage Systems and Treatment Plants,” Design Manual, EPA/625/1-85/018, Cincinnati, OH, 1985.
- U.S. Peroxide, “Iron Salts – Ferric and Ferrous,” 2011, www.h2o2.com
- Beltran, F.J., Ozone Reaction Kinetics for Water and Wastewater Systems, Lewis Publishers, 2004.
- Lenntech, “Water Disinfection Application Standards (for EU),” 1998, www.lenntech.com.
- Drago, J.A. et al, “Municipal Wastewater Ozonation Practice in the United States: Past, Present and Future,” Ozone: Science & Engineering, Volume 32, Issue 1, pp. 43-55, 2010.
- Plasma Technics, Inc, “Plasma Block Product Line, Product Detail,” 2011, www.plasmatechnics.com.
- Terry, P.A., “Application of Ozone and Oxygen to Reduce Chemical Oxygen Demand and Hydrogen Sulfide from a Recovered Paper Processing Plant,” International Journal of Chemical Engineering, Volume 2010, Article ID 250235, 2010.

