
A team of researchers from the University of Arkansas, ETH Zurich and the U.S. Environmental Protection Agency (EPA) recently solved an age-old mystery regarding the composition of a compound known to exist in water treated with chloramines. In a paper published in November 2024 in Science magazine, this enigmatic compound was finally identified, classified and named: chloronitramide anion, a byproduct of chloramine decomposition. This discovery raises new questions for water treatment plants that use monochloramine for disinfection, many of which do so as an alternative to chlorine.
Chlorine, Monochloramine & Disinfection Byproducts
Municipal water treatment plants typically use chlorine or monochloramine as a disinfectant to remove illness-causing bacteria from drinking water. Chlorine was the go-to chemical for disinfection for nearly all treatment plants from the 1900s until the 1970s, when disinfection byproducts (DBPs) were discovered.1 DBPs are formed from the interaction of chlorine with organic matter in the source water. These compounds can cause harm to humans when ingested in levels above safe limits for an extended period. Damage to the liver, kidneys or central nervous system and an increased chance of cancer2 are among the potential adverse health effects.
To limit exposure of these DBPs to the public, the EPA implemented stage 1 and 2 DBP rules3 primarily targeting trihalomethanes (THM) with a maximum contaminant level (MCL) of 0.080 milligrams per liter (mg/L) and haloacetic acids (HAA) with an MCL of 0.060 mg/L. In the wake of these rules, municipalities either started pretreating their source water for organics or transitioned from chlorine to the more stable disinfectant monochloramine (a chlorine derivative). Because of its chemical stability, monochloramine forms fewer DBPs.
This allows plants to reduce the public health risk of exposure to DBPs without requiring pretreatment of the water for organics. Currently, approximately 113 million Americans are drinking water that is disinfected using monochloramine.4
Chloronitramide Anion: A Mystery Disinfection Byproduct Is Named
As DBPs were discovered and the industry switched from chlorine to monochloramine, there was a similar increase in the study of monochloramine’s reactions in water. Through the study of these reactions, an unidentified byproduct was found in water that had been treated with monochloramine. Due to analytical limitations at the time, this byproduct could not be speciated.4 However, thanks to the advances in analytical capabilities and the work of a team of researchers led by Julian Fairey of the University of Arkansas, this byproduct was recently identified as the chloronitramide anion.5 It is a chemical compound with the structure Cl-N-NO2-. This anion was detected in drinking water samples from U.S. drinking water systems that use chloramines but not in samples of drinking water treated without chlorine-based disinfectants.
Because of its recent speciation, it has an unknown health risk, and many in the industry are viewing it with an abundance of caution as potentially hazardous in drinking water.5 Current and future studies will be required to determine health risks (if the chloronitramide anion is deemed to be a health hazard by testing) and how to remove this compound from the water.
Possible Treatment Technologies for Chloronitramide Anion
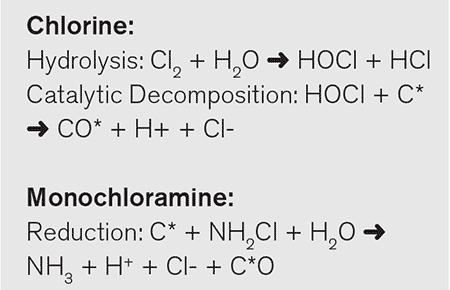
Activated carbon filters have been widely used to catalytically break down chlorine and monochloramine in drinking water. Please see the reaction schematics for this catalysis below. In these reactions, the C* is the activated carbon reacting in a catalytic manner.
These reactions change chlorine and monochloramine into chloride and ammonia, which are then released back into the water with no deleterious health impacts on the consumer. Regarding the chloronitramide anion, however, it is currently unknown whether activated carbon will have a similar catalytic effect.
While studies of activated carbon and chloronitramide will undoubtedly take place, it appears doubtful that activated carbon will break down this anion in a similar manner to monochloramine. The chloronitramide anion is a charged species that is soluble in water. Both characteristics limit the adsorption of this compound onto the activated carbon. This initial step of adsorption is required with chlorine and monochloramine for catalytic breakdown.
Contrary to how activated carbon reduces contamination in water, ion exchange appears to be a much more viable option for the reduction of the chloronitramide anion. Strong base anion exchange resins are used to reduce the concentration of anions present in the water. Currently this is being used for the reduction of sulfate, chloride, nitrate and, most notably, per- and polyfluoroalkyl substances (PFAS). Ion exchange resins are produced by binding functional groups to a plastic backbone (commonly polystyrene or acrylic). These functional groups have charged species on the end that attract counter ions. For example, nitrate-removing resins have an amine functional group attached to their backbone. When supplied by the manufacturer, this amine functional group has a counterion (in this example, assume it is chloride). This chloride ion then exchanges with nitrate in the water. The structure of the functional group of the ion exchange resin is more selective for nitrate than chloride, therefore facilitating this ion exchange. This selectivity can be tuned by altering the functional group and counter ion to target specifically charged or sized contaminants in the water.
The charge of the chloronitramide anion that posed a problem for adsorption onto carbon may be the reason it can be removed by a strong base anion exchange resin. Future testing will need to be conducted to determine the feasibility of this removal as well as the cost effectiveness. Interestingly, with the surge in public concern around PFAS compounds (also reduced by a strong base anion resin) and the EPA’s 2024 finalization of MCLs for PFAS at public drinking water plants, the introduction of another anion contaminant could offer an opportunity for cost-effective, multicontaminant removal using ion exchange in point of entry and point of use systems.
Regarding another commonly applied water treatment option—reverse osmosis—there is still debate as to whether the chloronitramide anion can be sufficiently reduced using this technology.
Future Studies for At-Home Treatment Options
As noted in the previous removal section, future studies are needed, and this is true for all commonly applied at-home water treatment methods as well. Currently, these studies are delayed due to the unavailability of standards to make synthetic waters with chloronitramide anion, lack of standardized detection methods and lack of knowledge of concentrations of this anion at the tap. The initial study by Fairey and his research team indicated that in a sample size of 40, a median concentration of 23 micrograms per liter (µg/L) was measured with the third and first quartile ending at 92 and 1.3 µg/L respectively.5
More studies will need to be conducted, first to determine if this compound is hazardous. If the compound is deemed hazardous, subsequent studies will be needed to identify the prevalence at the consumer tap. Finally, further research by the public and private sectors will need to be conducted around the reduction of the contaminant by current treatment methods for both efficacy and cost effectiveness for the homeowner.
References
DeMarini DM. A review on the 40th anniversary of the first regulation of drinking water disinfection by-products. Environ Mol Mutagen. 2020 Jul; 61(6):588-601. doi: 10.1002/em.22378. Epub 2020 Jun 19. PMID: 32374889; PMCID: PMC7640377.
Water, E. (n.d.). Disinfection Byproducts—Chlorination of drinking water. Washington State Department of Health. doh.wa.gov/community-and-environment/drinking-water/disinfection/disinfection-byproducts
Stage 1 and Stage 2 Disinfectants and Disinfection Byproducts Rules | U.S. EPA. (2024, July 8). U.S. EPA. epa.gov/dwreginfo/stage-1-and-stage-2-disinfectants-and-disinfection-byproducts-rules
International research collaboration identifies previously unknown chemical compound in drinking water | U.S. EPA. (2024, December 17). U.S. EPA. epa.gov/sciencematters/international-research-collaboration-identifies-previously-unknown-chemical-compound
Julian L. Fairey et al., Chloronitramide anion is a decomposition product of inorganic chloramines. Science386,882-887(2024).DOI:10.1126/science.adk6749

